The true cost of antibiotic resistance in Britain and around the world
When Kirsten Lavine went into hospital three years ago, it was for a routine procedure. She was having a hysteroscopy, where doctors feed a thin telescope into the womb to check for abnormalities. Thousands are performed across the country every year and Kirsten was home within hours.
That night she felt a searing pain in her abdomen. She took painkillers but after a sleepless night she went back to hospital, where things went from bad to worse. Her skin became mottled and she was breathless. She was just 43 years old.
Doctors realised she had an infection in her bloodstream and was going into septic shock. She was rushed to intensive care but her blood pressure dropped and her organs began to shut down. She was fed intravenous antibiotics and put into an induced coma, but the infection raged on.
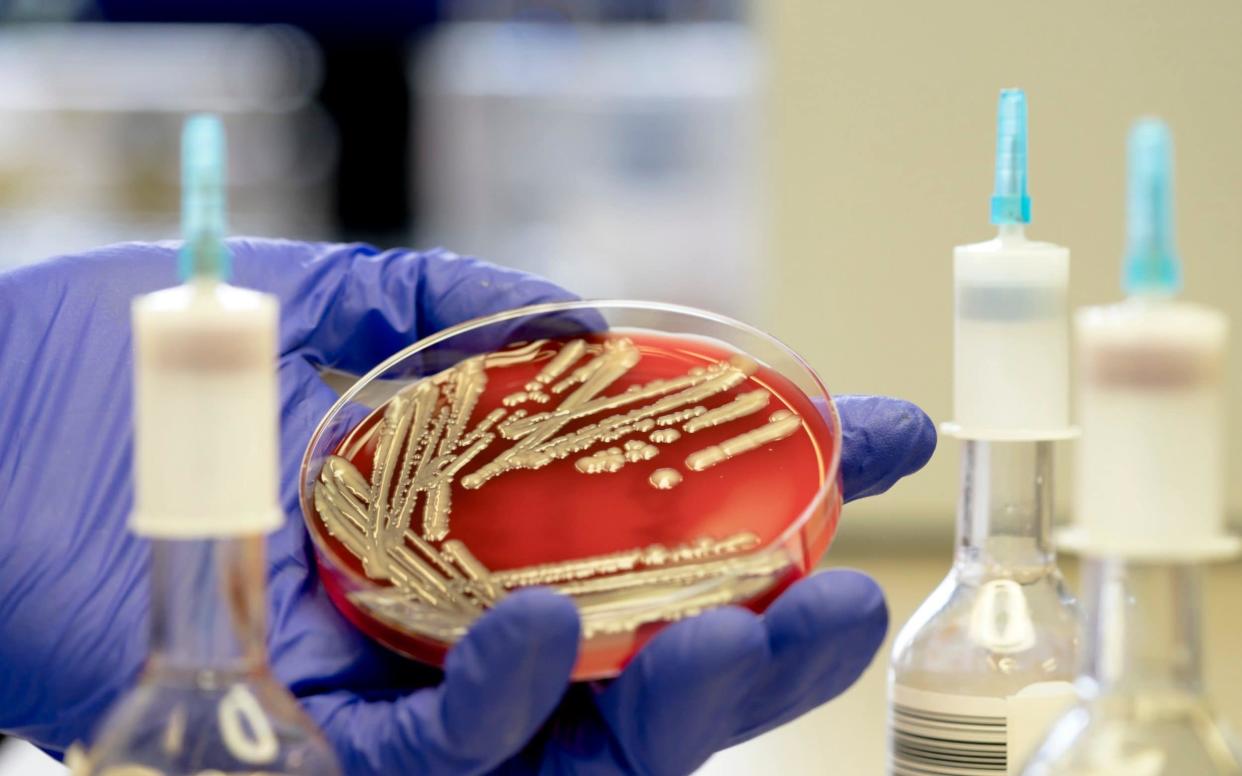
Her partner was told she may not live. She wasn’t responding to antibiotics, said the doctors, because the infection was from a strain of E. coli bacteria which was resistant to drugs.

This is no longer a rare occurrence. The increasing use of antibiotics both in medicine and farming around the world is constantly causing bacteria to mutate and become resistant to drugs.
Although the latest data show the steepest rises in consumption are in developing countries, the problem is a global one as so-called super-bugs rapidly spread across the world through international travel, trade and tourism.
And it’s not only bacterial infections which are becoming resistant but viruses like HIV, fungal infections such as thrush and parasitic infections like malaria. All over the world doctors are finding drugs used to treat these conditions – called antimicrobials – are failing.

Official figures suggest antimicrobial resistance (AMR) claims the lives of 5,000 people a year in the UK, though experts have argued the real figure is at least double that. Getting global figures on the problem is difficult but reliable estimates suggest 700,000 are already dying each year – one person a minute.
If no action is taken the death toll could rise to 10 million a year by 2050, a government-commissioned report, chaired by ex-Goldman Sachs economist and finance minister Lord Jim O’Neill found. This is more people than currently die of cancer.
Age is no barrier, with resistant infections suffered by the young and fit as well as the frail. No doctor can predict when an infection will be resistant. Sir Bruce Keogh, a cardiac surgeon who recently stepped down as medical director of the NHS after a decade, said he, like many NHS doctors and nurses, had seen patients die because of resistance, leaving him with a “deep sense of futility and hopelessness”.
I’ve watched patients deteriorate in front of my eyes because the germs are resistant ... People think of this as a problem of the future but it is a problem now
Sir Bruce Keogh
In a typical scenario a patient would be operated on and then develop an infection. Antibiotics would be given but the patient would not respond because the bug was resistant. By the time a more powerful antibiotic could be administered the patient – already weakened by surgery – would die.
“I’ve watched patients deteriorate in in front of my eyes because the germs are resistant. It’s a terrible thing for the patient and the family and all concerned.
“People think of this as a problem of the future but the reality is it is a problem now and it’s likely to get a lot worse in years to come,” said Sir Bruce.
The fallout doesn’t only come in deaths. Drug resistance means people suffer infections for longer than if the bugs could be killed with the usual course of antibiotics. This can result in devastating and disabling medical complications like amputations, brain damage and impairment of vital organs.

Resistance also causes repeat visits to GPs, longer stays in hospital and treatment with more expensive drugs. One super-bug outbreak in Manchester has cost a single hospital trust £8.4 million and is still ongoing. The World Bank has estimated it could cost the world economy $1 trillion every year after 2030.
So how did we get to a place where a common graze or a urinary tract infection could prove fatal? Who and what is feeding it? And how can we fight back?
Life-saving drugs
Antibiotics are actually a relatively new medical phenomenon – not much older than the NHS itself. Right up until the 1940s, when drugs such as penicillin and arsphenamine started being mass produced, hospital wards were full of people dying of common infections.
Antibiotics revolutionised medicine, and arguably society. They kept soldiers fighting on the front line in the war and some argue they fuelled the 1960s sexual revolution as much as the Pill because they removed the fear of catching syphilis.
In medicine, antibiotics allowed for procedures like joint replacements, organ transplants, bowel surgery, Caesarean sections and chemotherapy for cancer. Without them, the threat of infection would be too high. Some hospitals in Italy which are plagued with high rates of multi-drug resistant bugs have already had to suspend bone marrow transplants.

Borders are no protection
The only way to stop resistance developing is to expose microbes to crucial antimicrobials only when absolutely necessary.
In the UK there has been a concerted effort to reduce unnecessary or inappropriate antibiotic use in the healthcare system, with incentivised targets introduced in GP practices and hospitals to halve inappropriate prescribing by 2020.
The government has also launched a public awareness campaign asking people not to pester their GP for antibiotics and to take them as directed.
Across primary care prescriptions fell by 5.1 per cent between 2011 and 2016, though they rose within hospitals.
However, there is still significant variation in antibiotic use across the country and a recent Public Health England report found a fifth of prescribing by GPs is still inappropriate.
While some progress has been made in the UK and across other western European countries, the global picture is alarming, as the data released earlier this week attests.
In many countries weak regulation means it is still possible to buy antibiotics and other antimicrobials over the counter with no diagnosis or prescription, leading to inappropriate consumption.
The position is made worse by pharmaceutical companies which typically abide only by local rules rather than the much tougher international recommendations and standards.

In India, often dubbed the epicentre of the antibiotic resistance crisis due to unchecked consumption of the drugs, the Telegraph found that it was possible to purchase antibiotics of any sort, in a single packet or in bulk, without a prescription in any chemist.
As a result the country has soaring drug-resistance rates and the resistant bugs are being spread round the world.
David Partridge, a microbiologist in Sheffield and spokesperson for the British Infection Society, said: “In an ever shrinking world, we will continue to be exposed to resistant bacteria that have emerged overseas.”
One recent study found that, in central Birmingham, infection rates for a resistant bug called ESBL were as much as three times higher than other parts of the UK because of the city's links to South Asia.
Another issue is a gene called New Delhi metallo-beta-lactamase 1 (NDM-1), which makes bugs resistant to carbapenem antibiotics. It has been dubbed “nightmare bacteria” by the US Centers for Disease Control and Prevention (CDC) because half of people who develop a bloodstream infection die.
It was first found in a patient who acquired it in India in 2008, and has since spread all over the world, with more than a thousand cases recorded in Britain.

Tim Walsh, professor of medical microbiology and antibiotic resistance at Cardiff University and one of the researchers that identified the problem, said: "India is really the perfect storm because it is an economic powerhouse, lots of people do trade with India, yet it keeps quiet on these really important medical issues, which the rest of the world really need to know about."
While antibiotics are overused in human medicine, there is also staggering use in agriculture. Some 70 per cent of the global consumption of the drugs are used in animal and fish farming and to spray on crops. Some 131,100 tons were used on farmed animals in 2013, with this amount expected to rise by 53 per cent by 2030 as rising incomes mean more people eat meat.
The UK reduced antibiotic use in farmed animals by 27 per cent between 2014 and 2016, meeting the government target of 45 mg of drugs per kg of meat. But like the situation with human drugs, the reductions in Europe have not been replicated across the world.
In many countries, especially in Asia, the drugs are not always used to treat sick animals. Giving small doses to livestock can make them grow fatter, so more can be taken to market quickly. They can also be given en masse to whole herds, flocks or shoals to pre-emptively prevent disease, acting as a surrogate for good animal husbandry practices.
These practices have been illegal within the EU for more than a decade because they are known to fuel resistance but continue around the world.
Big Pharma and superbugs
Another major driver of AMR is waste from the pharmaceutical factories which manufacture the drugs. This process can release antibiotic effluent into the environment, where it causes superbugs to form and spread in watercourses and soil.
Most of the world’s antibiotics are made by a cluster of companies in India or China, where the problem of antibiotic waste has been repeatedly documented, although there have been examples in Europe too. In industrially polluted waterways, several studies have reported levels of antibiotics that are considerably higher than those found in the blood of people taking the medicine.
“Antibiotics in the environment do not do any good, they only contribute to risks,” said Professor Joakim Larsson, of the University of Gothenburg, who has been studying antibiotics in the environment for more than a decade.
No new drugs
While resistance among many pathogens is soaring, the pipeline for new drugs is near-empty. Of the 51 in clinical development, the World Health Organization says only eight will add value to the current arsenal of antibiotics.
In the 1950s and 60s many new classes of antibiotics were discovered, which could replace the drugs which were failing due to resistance. However, since 1987 only one new class of antibiotic has been discovered, with scientists making tweaks to existing classes instead.
The reason for this is that developing new antibiotics is not seen as profitable for pharmaceutical companies. It costs hundreds of millions to develop new drugs, and the opportunity for volume sales is limited by the problems of resistance.

Ron Daniels, a consultant in intensive care and anaesthetics and CEO of the UK Sepsis Trust, said the thought of no new antibiotics in the pipeline was "terrifying".
“It’s obscene that we are not seeing significant central development in the new classes of antibiotics and better understanding of their use,” he said. “Unless we develop new classes of antibiotics then antimicrobial resistance is quite likely to cause the global population to dwindle in the not too distant future, probably in our children’s lifetimes.”
Hope for the future
The government-commissioned O’Neill report, published in 2016, proposed a whole raft of measures to tackle the problem of AMR. This included creating new models of payment to incentivise pharmaceutical companies to develop new drugs, which the government is now exploring with the industry.
A £10m fund, the Longitude Prize, is now running, awarding groups trying to create a simple test that can tell the difference between viral and bacterial infections that can be used in developing countries. At a recent event on AMR in Westminster, Lord O’Neill said improved diagnostics would be a ‘gamechanger’: if doctors could identify the cause of infection it would lead to targeted treatment and reduce inappropriate use of antimicrobials.

Many companies are also coming up with promising alternatives to antibiotics, from probiotics, which strengthen the immune system and promote the growth of ‘good bacteria’; phage therapy, which uses viruses to kill bacteria; and gene editing software which chops out resistance genes from bacteria’s DNA.
The Fleming Fund, a £285m investment to improve surveillance and strengthen laboratories in low and middle income countries, has been launched and should provide clearer data on global patterns of resistance. The WHO has also charged countries with coming up with action plans to combat AMR, although is unclear how these plans will be resourced in poor countries suffering HIV and TB epidemics.
For some, however, many of these actions are already too late. Michael Weinbren, a microbiologist in Derbyshire, said doctors at the coalface are battling with resistance today.
“The real scare story is that patients are coming in with UTIs [urinary tract infections], which most people think aren’t a big deal,” he said. “I’ve heard of patients dying from a UTI because we had nothing left to give them.”

Kirsten Lavine thankfully did not die from the sepsis, but she came close. After microbiology tests revealed her infection was resistant doctors switched her to last line antibiotics – meropenem and vancomycin – she slowly began to recover.
The infection left her with long term damage to her small bowel, and it took her a year to fully recover. She wants to share her story to make others aware of the devastating impact sepsis and resistant infections can have.
She said: "One minute I was perfectly well and the next minute I was nearly dead, after a routine procedure."
Madlen Davies is a reporter with the Bureau of Investigative Journalism
Protect yourself and your family by learning more about Global Health Security

 Yahoo News
Yahoo News 
