Fears US regulators could rush out Covid-19 vaccine in time for election

Fierce debate has erupted in the US over whether health regulators should use their emergency authority to clear a coronavirus vaccine before it is formally approved, amid concerns the process may be rushed out before the presidential election.
Some scientists have expressed concerns that the Food and Drug Administration (FDA), which would need to approve any potential vaccine, could be pressured by the White House into clearing an unproven drug before election day on November 3.
It emerged this week that the Centers for Disease Control and Prevention (CDC) had told state governors and health officials to be ready to distribute a possible coronavirus jab by November 1, earlier than predicted.
Professor Peter Hotez, dean of the National School of Tropical Medicine at the Baylor College of Medicine, Texas, told the Telegraph he worried about the risks of giving an expedited vaccine to millions of healthy Americans.
"The concern is that we rush this process without understanding the effectiveness or safety profile of the vaccine. If something unexpected happens, it could undermine confidence in the US Covid-19 programme," he said.
"We have a terrible anti-vaccine, anti-science movement in America. The anti-vaxxers are waiting in the wings for this like lions surrounding an injured impala on the savannah."
The FDA last week used its emergency powers to approve the use of convalescent plasma, where blood cells are taken from patients who have recovered from Covid-19 and developed antibodies against the virus and then infused in sick patients.
The treatment had been touted by President Donald Trump as a cure for the novel coronavirus. However, the National Institutes of Health criticised the FDA’s decision, saying there was “insufficient data” to show convalescent plasma works.
“It’s hard not to see this as a push for a pre-election vaccine,” said Saskia Popescu, an infection prevention epidemiologist at the University of Arizona.
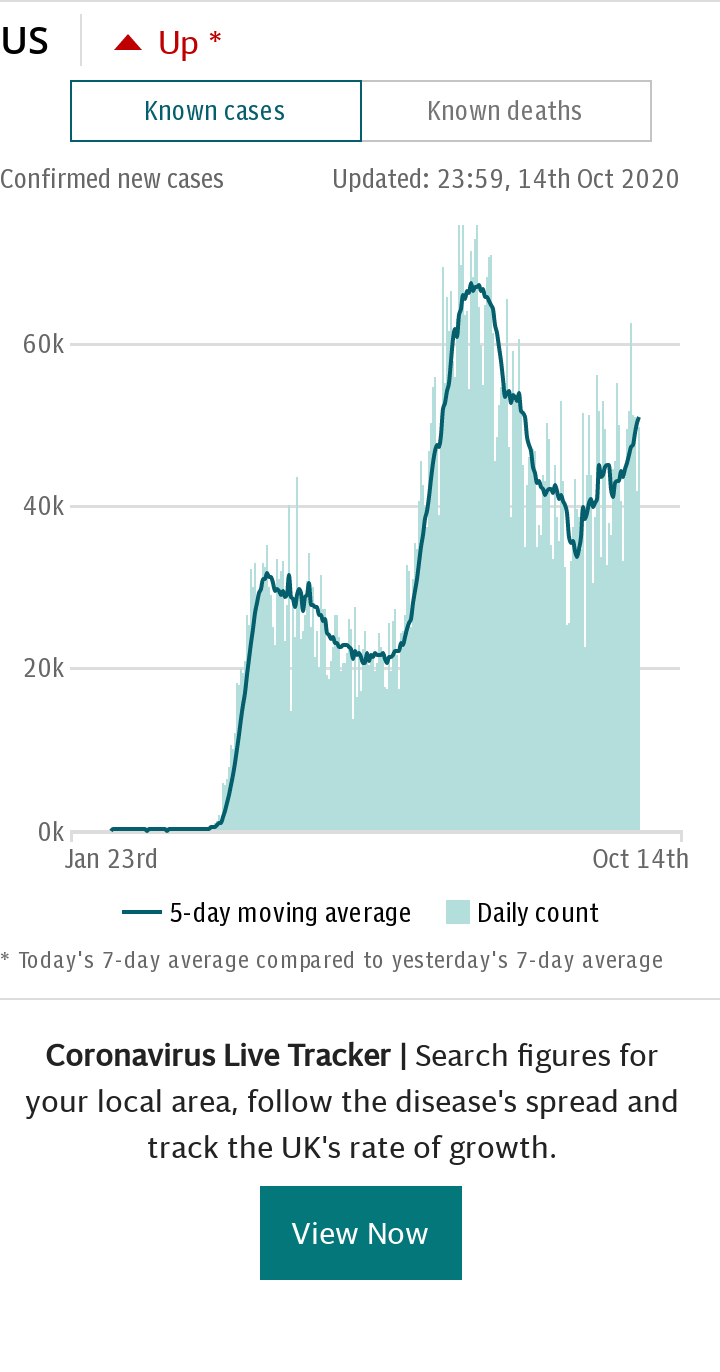
Mr Trump’s repeated promise to deliver a vaccine this year, “or maybe even sooner,” has become central to his reelection campaign. Approval ratings on his handling of the coronavirus outbreak plummeted amid a new surge in cases and a rise in deaths, which have now reached 186,000.
There are two potential vaccine candidates currently being trialled in the US: Moderna and Pfizer. In their earlier human studies, neither vaccine produced serious side effects.
Pfizer recently said it was “on track” for seeking government review “as early as October 2020.” Moderna has said it expects to complete enrollment in its Phase 3 trial in September, but has not provided an estimate about when the vaccine might be ready for the public.
Phase 3 is the last stage before drugmakers can seek a green light from regulators.
Dr Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said on Thursday it is “conceivable” that the US could have a vaccine by late October, as Pfizer predicted, “though I don't think that that's likely.”
“If you look at the enrollment and the kind of things you need to ensure the vaccine is safe and effective, most of us predict November, December, by the end of the year. (October) is unlikely, but not impossible," he told CNN.
The nation’s leading infectious disease doctors have demanded that vaccine safety and effectiveness data be “reviewed by internal, as well as independent experts,” if the agency uses an emergency use authorisation process.
Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, which oversees vaccines, tried to dispel concerns this week, saying the agency will insist on the same safety and efficacy standards for an authorisation as for a full approval.
“Our intention … is not to give the American public anything less than a gold standard,” he said.
However, as it was a public health emergency he said it would be unethical to demand all the paperwork usually required for a full approval.

 Yahoo News
Yahoo News 