Hundreds of patients lose battle over allegedly 'defective' hip implants

Two thirds of lead claimants given ‘faulty’ hip replacements “did not suffer an adverse reaction to metal debris” a judge has ruled, as she rejected one of the largest group actions to appear before the High Court.
Mrs Justice Geraldine Andrews said DePuy’s metal-on-metal Pinnacle Ultamet products has not been ‘defective’, and could not be blamed for the appearance of “abnormal” immunological responses in some patients.
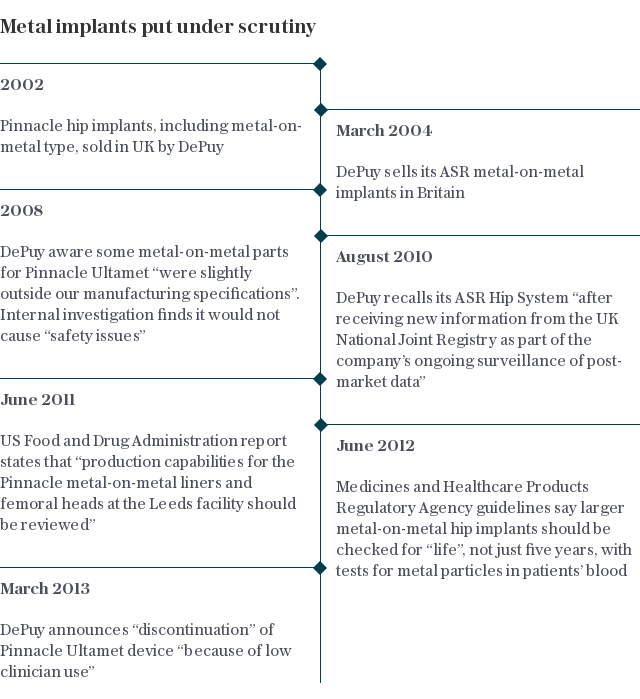
More than 40,000 patients in Britain have received a “metal-on-metal” hip replacement since their introduction in the 1990s.
Around 23,000 of the devices fitted since 2003 were types made by DePuy, part of medical giant Johnson & Johnson.
Hundreds more metal-on-metal claims against a number of other manufacturers were put on hold pending the outcome of the trial, in which a total of 312 claimants brought contested action against the device’s American manufacturer through solicitors solicitors Leigh Day.
They argued that recipients of the cup-and-ball system were injured as a result of the early failure and consequent revision surgery of their metal-on-metal prosthetic hips, caused in part by ill-fitting joints and the release of toxic metals particles following “normal use”.

However, Mrs Justice Andrews said the claimants had failed to prove that the Pinnacle 36mm metal-on-metal prosthesis “did not meet the level of safety that public were entitled to expect at the time when it entered the market in 2002”, and that four out of six lead claimants were not directly affected by it.
She concluded that there was no “materially greater” risk of the joint failing within the first 10 years after implant than with another, comparable prosthesis, or that the product carried with it an ‘abnormal risk’ of damage as alleged. DePuy was therefore not liable for the claims made against it.
Mrs Justice Andrews was also critical of the National Joint Registry (NJR) - the public body that holds information on all hip, knee, ankle, elbow and shoulder joint replacements across the NHS and independent healthcare sector in England, Wales, Northern Ireland and the Isle of Man, and is relied upon by surgeons, manufacturers and regulators to make decisions over product safety.
She said the NJR’s data was not “sufficiently reliable” leading to “far too much guesswork involved in trying to factor in all the variables” required to draw direct comparisons between the way different joints performed.
A spokesman for Leigh Day said the firm was “extremely disappointed” by the judgement, and was “in touch with our clients to discuss what next steps could be taken.

“This is a complex and lengthy judgment, the implications of which are being carefully considered, however, the impact it will have on consumer safety and the ability of consumers to get redress, cannot be underestimated”.
Samantha Silver, of law firm Kennedys who acted for DePuy, said Monday’s decision would provide manufacturers with confidence that the courts “can take into account the benefits and the inherent risks of certain products, for example, in cases where there is a known side effect or complication and the overall benefits outweigh the risks.
"The decision that the safety of new products should be compared to products existing at the time they are introduced to the market also safeguards the position of those developing new, potentially life-enhancing, technologies in the future."
NJR director of operations Elaine Young said: "NJR data collection commenced in April 2003 for England and Wales. Compliance with data entry to the NJR was initially low in the early years.
"For the last three years the NJR has undertaken a comprehensive annual data quality audit across all hospitals submitting NJR data and are now confident that the NJR is a robust and reliable source of information to be used for patient safety".
Norman Lamb, the former Liberal Democrat health spokesman, said: “The human impact of failing replacement hips with this problem of debris has been enormous for a very many people and they will feel devastated by this judgement".

 Yahoo News
Yahoo News 