People with significant allergies told not to get vaccine after two NHS workers suffer reaction
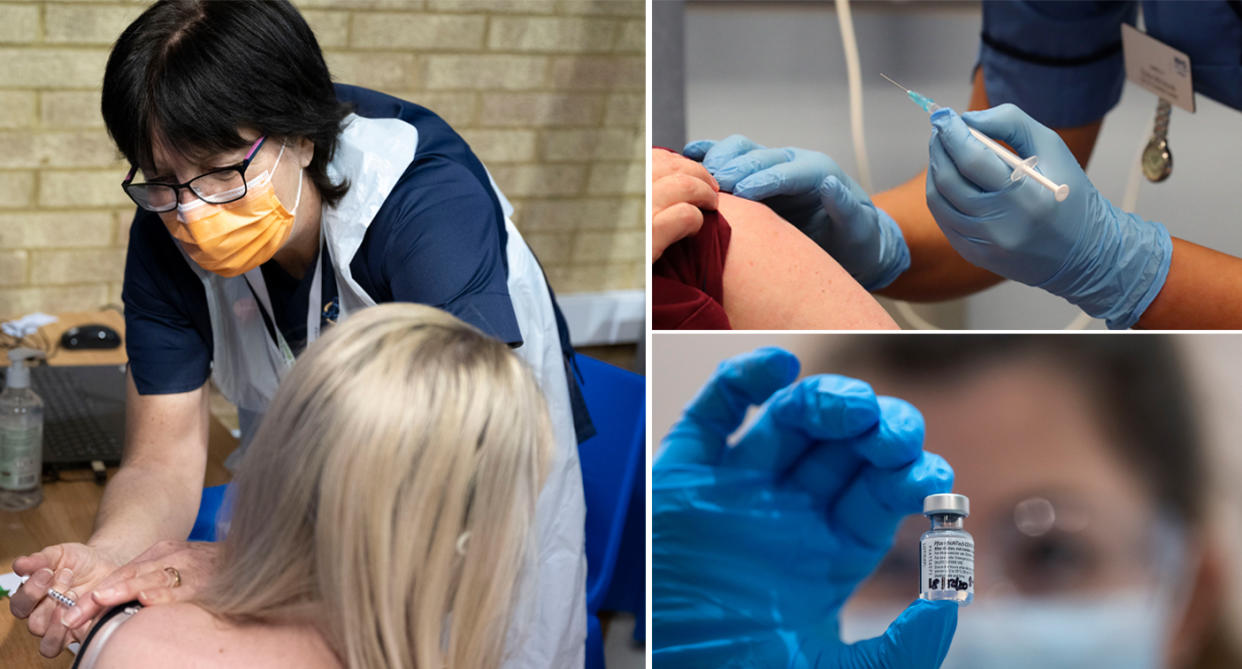
People who have a history of “significant” allergic reactions should not currently get the Pfizer-BioNTech coronavirus vaccine, the UK regulator has warned.
It comes as two NHS staff members who had the jab on Tuesday had allergic reactions. Both are understood to be recovering.
The NHS in England said all trusts involved with vaccinating have been informed.
The Medicines and Healthcare products Regulatory Agency (MHRA) gave precautionary advice to the NHS that anyone with a history of “significant” allergic reactions to medicines, food or vaccines should not get it.
It is believed hundreds of people were vaccinated across 70 hospital hubs in the UK on Tuesday.
Watch: First COVID-19 vaccine given to patient in UK
Health staff, care home workers and elderly people were first in line to receive an initial shot of the Pfizer-BioNTech vaccine, which arrived in the UK last week.
Professor Stephen Powis, national medical director for the NHS in England, said: “As is common with new vaccines, the MHRA have advised on a precautionary basis that people with a significant history of allergic reactions do not receive this vaccination after two people with a history of significant allergic reactions responded adversely yesterday.
“Both are recovering well.”
MHRA advice added that resuscitation facilities “should be available at all times for all vaccinations”.
It is understood that both staff members have a significant history of allergic reactions and need to carry an adrenaline auto injector with them.
They developed symptoms of “anaphylactoid reaction” shortly after being jabbed and have recovered after treatment.
An initial 800,000 doses of the vaccine are available, enough for 400,000 people because each patient requires two doses.
A total of 40 million doses have been ordered so far – enough for 20 million people – but the bulk of vaccinations are not expected to be carried out until next year.
Matt Hancock became visibly emotional on Tuesday – or V-Day, as he called it – as the first groups of people started to receive the jab.
“And there’s still a few months to go, I’ve still got this worry that we can’t blow it now,” he told ITV’s Good Morning Britain.
“We’ve still got to get the vaccine to millions of people, so we’ve got to keep sticking by the rules, but there’s so much work has gone into this and it makes me proud to be British.”
Read more
The Tier 3 COVID lockdown rules explained
The Tier 2 COVID lockdown rules explained
The MHRA became the first regulator to approve the Pfizer-BioNTech vaccine last week, which Dr June Raine, the agency’s chief executive, said was due to the “flexibility and agility” of clinicians and scientists.
“It is my understanding that the [US] Food and Drug Administration will be looking this week to conclude their review, and the European Medicines Agency fairly shortly thereafter,” she told the Commons Science and Technology Committee on Wednesday.
Watch: The dos and don’ts of a COVID Christmas

 Yahoo News
Yahoo News 
