Vaccine race: Test subjects could be exposed to Covid in January
Test subjects could be exposed to the new coronavirus in controlled settings from January in a bid to speed up vaccine development, officials have confirmed.
The Government is backing so-called human challenge studies, whereby a small number of participants will be purposefully exposed to Covid-19.
It is hoped these studies will help speed up vaccine development.
A small number of young and healthy participants – aged 18 to 30 – will be recruited to take part in the trial.
This group of up to 90 participants will then be exposed to the virus in a controlled environment.
They will be carefully monitored to establish the smallest amount of virus it takes to cause Covid-19 but not serious illness.
It is hoped the trials will start in January, with results expected by May 2021, pending approval from regulatory bodies and ethics committees.
The Government announced that it is investing £33.6 million to back the studies in partnership with Imperial College London, hVIVO and the Royal Free London NHS Foundation Trust.
The aim of the research will initially be to discover the smallest amount of virus it takes to cause Covid-19 infection in small groups of healthy young people, who are at lowest risk of harm. The next phase will be to test vaccines.
The studies will be carried out under strict conditions at the Royal Free Hospital in London and will feature healthy young adults, carefully selected by researchers, who will be compensated for their involvement.
After the initial study, the volunteers will be tracked for a year.
Business Secretary Alok Sharma said: “We are doing everything we can to fight coronavirus, including backing our best and brightest scientists and researchers in their hunt for a safe and effective vaccine.
“The funding announced today for these ground-breaking but carefully controlled studies marks an important next step in building on our understanding of the virus and accelerating the development of our most promising vaccines which will ultimately help in beginning our return to normal life.”
Lead researcher on the human challenge study Dr Chris Chiu, from Imperial College London, said: “Our number one priority is the safety of the volunteers. No study is completely risk-free, but the Human Challenge Programme partners will be working hard to ensure we make the risks as low as we possibly can.
“The UK’s experience and expertise in human challenge trials, as well as in wider Covid-19 science, will help us tackle the pandemic, benefiting people in the UK and worldwide.”
Dr Chiu said that he and his team are proposing to use remdesivir as a rescue treatment for the volunteers, “so that means we will give it to people as soon as we start detecting the viral infection likely before the onset of any symptoms”.
He added: “We believe that giving it very early in the course of the infection will limit the spread of the virus into the respiratory tract as well as into the lungs and and prevent those more severe systemic features of Covid-19.”
Dr Chiu said that while remdesivir has been shown to have no effect on hospital patients with severe Covid-19 in recent studies, the human challenge studies are going to be “a very different situation” as “our participants will not be sick – they will be very early in the course of their infection”.
However, he added that it was unclear at present whether remdesivir will interfere with the ability to detect efficacy of vaccines.
Kate Bingham, chairwoman of the Government’s Vaccine Taskforce, said: “This research will improve understanding of the virus, the biology of the disease, the signs that a person is protected from infection or developing the disease, the vaccine candidates, and will help in making decisions about research, that it is carried out safely and based on up-to-date evidence.
“There is much we can learn in terms of immunity, the length of vaccine protection, and reinfection.”
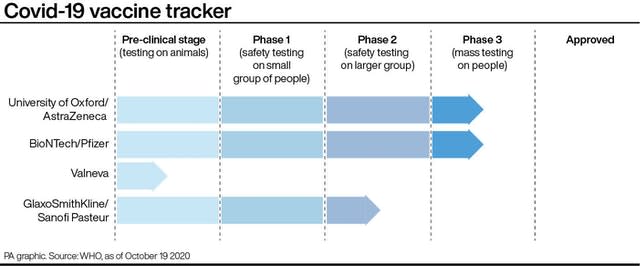
England’s deputy chief medical officer, Professor Jonathan Van-Tam, said: “First, for the many vaccines still in the mid-stages of development, human challenge studies may help pick out the most promising ones to take forward into larger phase three trials.
“Second, for vaccines which are in the late stages of development and already proven to be safe and effective through phase three studies, human challenge studies could help us further understand if the vaccines prevent transmission as well as preventing illness.”
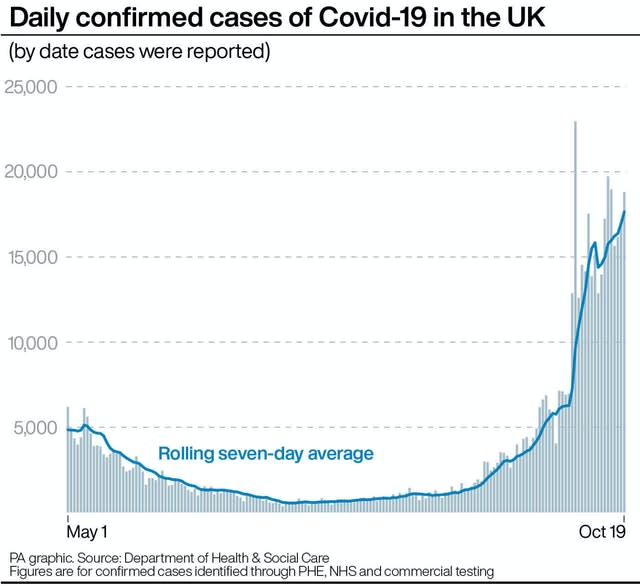
Peter Openshaw, from Imperial College London, said the initial part of the trial will involve exposing people to the virus to establish the lowest possible safe dose.
“Initially, until May next year, we are expecting to escalate the dose and ascertain the exact safety.
“Once that’s done we will be ready to actually test vaccines head to head and compare different vaccines,” he said.
Professor Openshaw, who is co-investigator of the project which will see humans exposed to Covid-19 in controlled settings, told BBC Radio 4’s Today programme that volunteers would be infected via the nose and then monitored “very carefully”.
“(These studies) are enormously informative because we can do such very, very careful monitoring under controlled conditions,” he added.
“The aim of these studies is not to make people ill, but to get the virus to replicate in the nose.
“We think that, by taking every precaution, we can really limit the infection and then we should be able to do it quite safely given the vast amount of experience we have in this field.”
The news comes after the Government’s chief scientific adviser said it is “unlikely” that a coronavirus vaccine will stop the disease completely.
Sir Patrick Vallance said that only one disease – smallpox – had ever been completely eradicated.
Giving evidence to the joint Commons and Lords National Security Strategy Committee, he said that, in future, treating Covid-19 may become more like seasonal flu.
Sir Patrick said that, over the next few months, it will become clear whether there are any vaccines that do protect, and how long for.
He added that, while a number of candidates cause an immune response, only phase three trials will indicate whether they stop people from being infected.
It comes as health officials announced the National Institute for Biological Standards and Control is joining the Coalition for Epidemic Preparedness Innovations’ global centralised lab network to evaluate Covid-19 vaccine candidates.
Health Secretary Matt Hancock said: “The UK is leading the way in developing a safe and effective vaccine for Covid-19 which will be absolutely crucial to saving lives and starting our return to normality.
“We have one of the best medical regulators in the world and I’m delighted the NIBSC will play a crucial role in developing international standards to help ensure all vaccines are of the highest quality, safety and effectiveness.”

 Yahoo News
Yahoo News 
