‘I’ve tried and tried, and I can’t get rid of it’: The women living with drug-resistant infections

Caroline Sampson, 60, contracted an antibiotic-resistant urinary tract infection (UTI) in 2016 after undergoing a minor gynaecological procedure. Almost seven years on with little respite, she says the condition has been life-changing.
Ms Sampson, introduced to The Telegraph through Antibiotic Research UK, is just one of many in the UK living with antimicrobial resistance (AMR), which has become a leading public health threat in recent years.
“We expect antibiotics to treat bacterial infections, but sometimes, that’s simply impossible,” Dame Jenny Harris, Chief Executive of the UK Health Security Agency (UKHSA), warned last week, emphasising the surge in antibiotic-resistant infections in the UK.
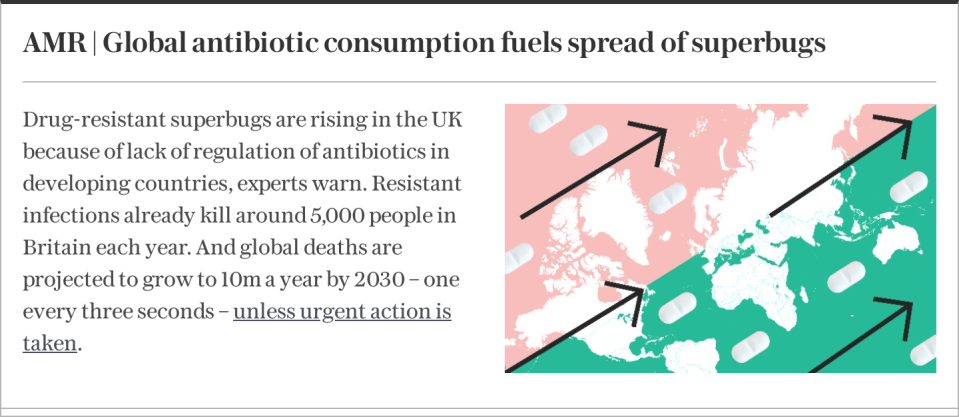
Most people think of antibiotic resistance as a worry for the future. Termed the ‘silent pandemic,’ projections show that antimicrobial resistance (AMR) could claim 10 million lives annually by 2050.
But AMR is very much here today. Globally, it has already superseded leading infectious disease killers, including malaria and HIV. In England alone, 58,224 people experienced an antibiotic-resistant infection in 2022, up 4 per cent from 2021.

Common antibiotic-resistant infections in the UK include MRSA (methicillin-resistant Staphylococcus aureus) and C.diff (Clostridium difficile) – both of which plague hospital wards and pose an especially acute risk to frail, often elderly, post-operative patients.
Other common bacterial infections, including UTIs, some forms of diarrhoea, and sexually transmitted infections have also become subject to high levels of drug resistance. These and other infections pose a particular risk to the young, and without effective treatment can spread and prove fatal.
Ms Sampson’s journey with antibiotic resistance began in 2016 when she was prescribed a three-day course of antibiotics to treat a UTI, which she developed after having a hormonal coil fitted by her GP.
The antibiotics failed to clear the infection, however, and it came back with a vengeance. “I’ve tried and tried, and I can’t get rid of it,” she said.
Over the past seven years, she has been prescribed more than 11 different oral antibiotics, to little avail. She even received an intravenous gentamicin injection, usually reserved for the most severe antibacterial infections, namely sepsis. But even that didn’t work.

Living with a resistant and chronic UTI has made Ms Sampson’s life extremely difficult. “I’m really limited in what I can do on a day-to-day basis,” she said.
Without an available treatment to ease her discomfort, she says her life has changed dramatically. Her plans are often cancelled due to constant pain and years of antibiotic treatment have led to debilitating side effects, including “destroying” her gut health and developing neuropathic pains in her legs, hindering her ability to walk.
The physical and mental strain of AMR eventually led her to quit her job at a major public school, a position she had held for some 25 years. “I’m homebound a lot of the time, and I don’t cope very well. Somedays are absolutely dreadful. It’s been heartbreaking.”
Vanessa Carter, 44, is also living with the fallout from AMR. An executive director, she battled a recurrent MRSA infection following a car crash that led to multiple reconstructive surgeries on her face.
A cheek prosthetic Ms Carter had implanted during one surgery became infected. Multiple debridement (cleaning) operations were performed in order to save the prosthetic and rid her of the bacteria, operations which subsequently failed.

She spent three years on different antibiotics, none of which seemed to work. The invasive MRSA infection then spread to the bone in her cheek. MRSA typically lives on the skin, if it gets into the bloodstream it can lead to sepsis.
“One mistake I made repeatedly was to quit a course of antibiotics halfway. I was ignorant of the risks of doing so,” she said. “That was contributing to my antibiotic-resistant infection worsening.”
After a challenging three-year battle with illness, a breakthrough emerged with the introduction of the last-resort antibiotic Vancomycin. While the medication proved effective in overcoming the infection, its aftermath has left Ms Carter scared physically.
Now a member of the recently-created World Health Organisation (WHO) Strategic Technical Advisory Group on AMR, Ms Carter is an advocate for patient education and resistance prevention. She also runs a patient-support charity for survivors, The AMR Narrative.
“Antibiotics are life-saving drugs, but we keep squandering them unnecessarily. Diseases that we once thought we had under control will threaten us again. From experience, nothing could be more frightening than feeling like you have lost the battle.”

There are a number of causes for AMR.
Human behaviour is a primary cause. Microbial resistance is a natural process but is heavily aggravated by excessive and incorrect use of drugs. When a patient does not finish their prescribed course of antibiotics, it allows some bacteria to survive and adapt, reducing the effectiveness of the drug in future.
Similarly, when antibiotics are used to treat a viral condition, it exposes bacteria to the drugs unnecessarily, allowing them an opportunity to become resistant.
This is particularly challenging in fragile health systems, where antibiotics are often under-regulated.
Bacteria can be spread through airborne transmission, water, food, animals, plants and humans; factors including poor sanitation and access to clean water can add significantly to the issue.
Agriculture also plays a significant role in AMR. The use of antibiotics in livestock for disease prevention and promotion of growth has notably contributed to the rise of resistant bacteria that can then spread to humans.
Neil Ward, a global genomics expert at PacBio, is calling for greater collaboration and sharing of genomic pathogens to bolster communication between health and policy bodies.
Specifically, Mr Ward argues that better investment into the genetic sequencing of agricultural threats will allow for stronger international biosurveillance, allowing scientists and officials to respond better to new antimicrobial threats. The WHO has echoed this, calling for “cross-sectional collaboration” from the agricultural sector, to the food industry, to health and pharmaceutical leaders.
Mr Ward suggests that government organisations, including the UK’s Department of Environment, Food & Health (DEFRA) could play a critical role in disease surveillance, considering that many pandemics have stemmed from microbial transmission from animals to humans.
In 2019, the UK government released a five-year plan to support a 20-year vision for the reduction of antimicrobial resistance. Key points include; reducing the need for unintentional exposure to antimicrobials through the environment including food, water, and animal infection; optimising the use of antimicrobials in healthcare settings in agriculture; and investing in new therapeutics, including vaccines.
Whilst 93 per cent of countries have established national action plans to tackle AMR, only 24 per cent report effective implementation. More worryingly, only 10 per cent are financed.
Protect yourself and your family by learning more about Global Health Security

 Yahoo News
Yahoo News 
