EU's coronavirus jabs 'may have ended up' in Britain

AstraZeneca vaccines meant for and paid for by the EU could have ended up in Britain, diplomatic sources in Brussels claimed today.
The suspicion is that the British-Swedish pharmaceutical company supplied the UK from the EU vaccine stock because Britain paid a higher price for the dose and approved it sooner.
On Monday, Brussels threatened to block EU vaccine exports to non-EU countries, after AstraZeneca revealed that it would not be able to fulfil its contractual obligations as originally hoped.
Ursula von der Leyen, the European Commission president, said on Tuesday that the EU would press on with the export mechanism that would force companies to ask for permission before vaccines could leave the bloc.
In a speech to the World Economic Forum in Davos, Mrs von der Leyen said, “Europe invested billions to help develop the world's first Covid-19 vaccines to create a truly global common good. Europe is determined to contribute to this global common good but it also means business.”
She added: “And now, the companies must deliver. They must honour their obligations and this is why we will set up a vaccine export to transparency mechanism.”
A European Commission spokesman said: "How worried are we about the state of vaccinations? Well, we are worried that is for sure. We are dealing with a very important pandemic and vaccination is very important."
The UK is dependent on the Pfizer vaccine, which is produced in Belgium, and is expecting almost 3.5million doses to be delivered in the next three weeks. That supply could be jeopardised if the EU decided to block the exports after the AstraZeneca controversy.
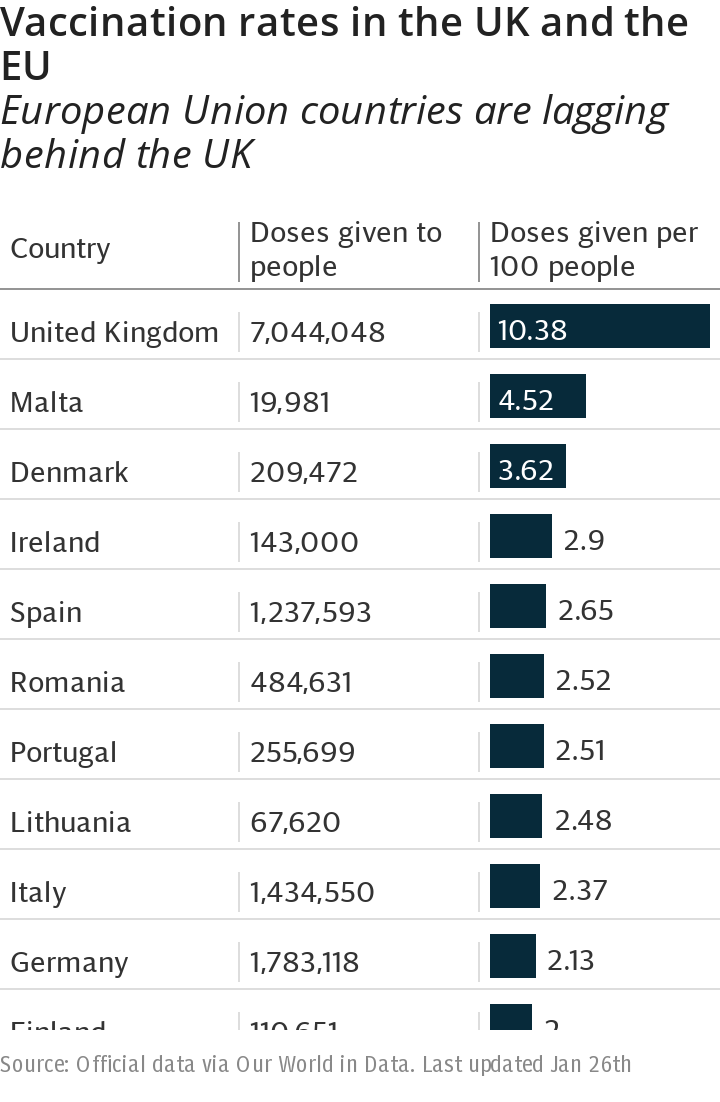
The EU, which negotiated as a bloc, reached an agreement for up to 400million doses of the AstraZeneca vaccine in August. The Reuters news agency reported that deliveries would be cut by 60 per cent in the first quarter of the year, a drop of 31 million doses.
The European Medicines Agency is expected to approve the vaccine at the end of this week and supplies were set to roll out soon afterwards in a boost to the EU’s vaccination strategy, which is lagging behind Britain’s.
In December, the Government said that four million doses of the vaccine developed by AstraZeneca and Oxford University would be delivered by the end of 2020. It also said that those supplies would be imported from AstraZeneca factories in Germany and the Netherlands because British plants, which now provide the whole UK stock, were hit by delays.
“There are people in Brussels who think that vaccines originally supposed to build up the EU vaccine stock and to be delivered to the EU after market authorisation have actually ended up in Britain,” said an EU diplomat.
“As long as AstraZeneca doesn’t come forward with an explanation about where the vaccine doses ended up, it is difficult to put an end to this suspicion.”
"We see that doses are being delivered elsewhere and we know we’ve signed an agreement with AstraZeneca in August, that member states placed their orders around October and we’re now at the end of January and therefore we believe the doses should be basically available to be delivered," the European Commission's chief spokesman said.
He denied the EU was guilty of "vaccine nationalism", which was an accusation levelled at Brussels by the UK vaccines minister Nadhim Zahawi.
"Europe is working and has been working since the beginning on playing a major role in helping countries with lower incomes than European Union countries to get access to the vaccine," he said.
AstraZeneca’s explanation in three calls with officials from the European Commission and all 27 EU member states on Monday - that there were production problems - was deemed “unsatisfactory” by the EU’s Health Commission. Officials grilled the AstraZeneca chief over why there were not similar supply problems in Britain.
Valdis Dombrovskis, the EU’s trade commissioner, said the bloc was not planning to impose an export ban at this stage. The commission hoped to finish a proposal for the new tool by the end of the week, he said.
“It is primarily an export transparency mechanism,” he said, “to bring clarity on manufacturing capacity.”
Jens Spahn, the German health minister, said the tool was about Europe's "fair share". "That is why it makes sense from my point of view that we have an export restriction," he said.
He said he was in favour of "vaccines leaving the EU needing a licence, so that we at least know what is being produced, what is leaving Europe and - if it is leaving Europe - whether there is then a fair distribution".
Watch: How does the number of coronavirus deaths in the UK compare with other countries?
The French MEP Veronique Trillet-Lenoir backed the European Commission plans and said AstraZeneca could have given EU supplies to non-EU countries paying a higher price.
She told the BBC Radio 4’s Today programme on Tuesday: “It could really be an explanation. The European Commission has been negotiating in the name of 27 states, which means it is possible to have lower prices.
“Other countries - including the UK but maybe also the US - are paying a higher price. It is their choice but should not enter into the firm’s decisions.”
Ms Trillet-Lenoir, a member of Emmanuel Macron’s party, said the EU was mulling legal action against AstraZeneca, as well as the measures to control exports.
She said: “The Commission is right to say that, when trust is betrayed, we should take strong decisions.”
The details of the EU contract with AstraZeneca are secret. The Berlaymonster website reported details of another advance purchase agreement with the US CureVac vaccine, which it said could be similar to the AstraZeneca deal.
The CureVac deal, as revealed to US regulators, recognised the risk of supply disruption and only called on the company to make “reasonable best efforts” to hit the targets.
That could limit the EU’s options for legal action if the AstraZeneca agreement was similar, Berlaymonster reported.
Watch: When will the lockdowns end in each UK nation?

 Yahoo News
Yahoo News 
