This FDA-Approved Coronavirus Test Gets Results in Minutes—but Is It Accurate?
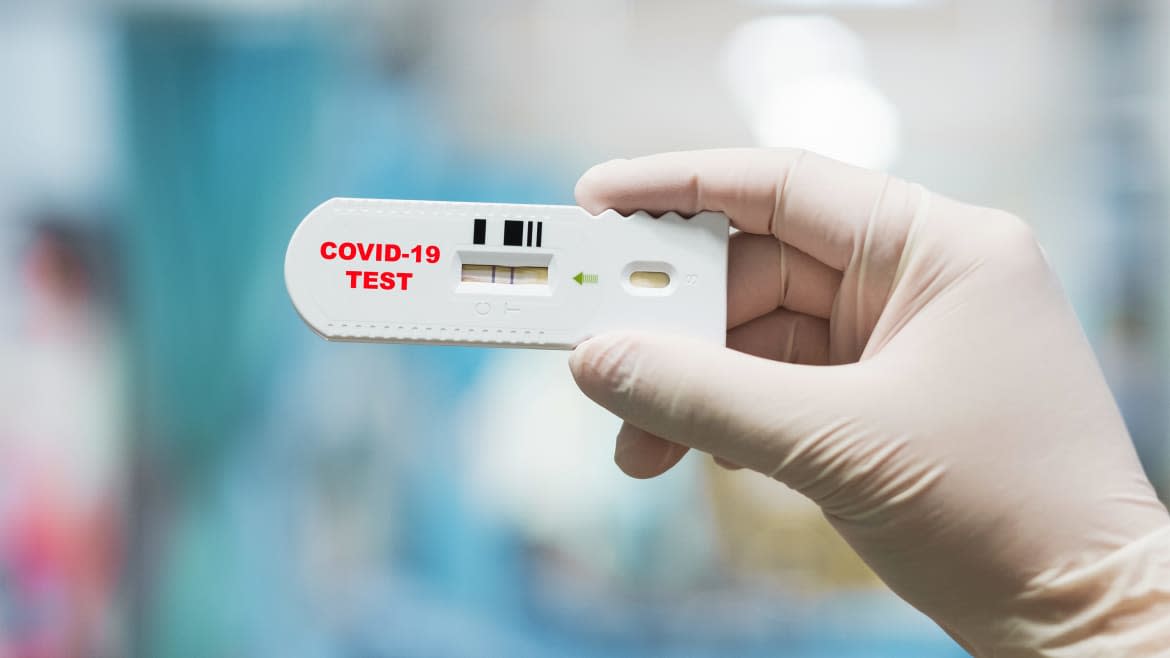
New Jersey pharmaceutical firm Becton, Dickinson and Company has won approval from the U.S. Food and Drug Administration for a fast, easy-to-use test for novel coronavirus infections.
At first glance, the Veritor test system from BD, as the company is widely known, might seem like exactly the thing public-health officials have been clamoring for since SARS-CoV-2 first reached the United States early this year.
But a closer look at BD’s data reveals limits to the new test’s effectiveness. And that underscores how difficult it can be to quickly develop a coronavirus test that’s both fast and accurate.
The FDA approved BD’s testing system for emergency use in a July 2 letter. The approval expires when the federal government ends its official state of emergency in response to the pandemic.
Is CRISPR the Solution to the Coronavirus Testing Dilemma?
The basic Veritor system, which retails for around $500, isn’t actually new. The medical industry already uses it to quickly test for flu infections. What’s new is the special “assay” for the novel coronavirus. You swab a patient, stick the sample in a vial containing a special cocktail of reagents, squirt the sample onto a plastic card then slip the card into a palm-sized test unit.
In 15 minutes or so, the unit’s digital display tells you whether it thinks the patient has the virus.
In theory, the rapid “point-of-care” test meets an urgent need. “We’re trying to make this something that’s not a whole lot more complicated than a pregnancy test,” Keith Jerome, a University of Washington virologist, told The Daily Beast in March.
But that’s easier said than done, Jerome stressed. Highly accurate testing usually requires complex equipment. “There’s a reason why we do it in a big, complicated lab,” he explained.
As it turns out, the Veritor machine with a coronavirus assay accurately detects the virus only 84 percent of the time, BD found after a trial involving 226 patients in 21 locations. Laboratory molecular tests, by contrast, are nearly 100-percent sensitive but can take days to complete.
“Negative results should be treated as presumptive, do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions,” the FDA warned.
Poor sensitivity doomed an earlier, 13-minute coronavirus test from Chicago-based pharma Abbott Laboratories that the FDA approved for emergency use in March. President Donald Trump even touted the test on the White House lawn.
But some users were disappointed. The Cleveland Clinic told NPR the Abbott test delivered a false negative nearly one time out of six. In April, the FDA issued revised instructions for the Abbott test that the agency hoped would improve the test’s sensitivity.
“The last point-of-care test by Abbott failed because it was not as sensitive as the standard tests,” Jeffrey Klausner, a professor of medicine and public health at UCLA who previously worked at the Centers for Disease Control and Prevention, told The Daily Beast.
The BD test could have the same problem, Klausner said.
The At-Home COVID Testing Revolution Is Here. Can It Deliver?
There’s another problem. The FDA only approved the BD test for patients “who are suspected of COVID-19 by their healthcare provider within the first five days of the onset of symptoms.” And the only labs allowed to use the new test are those that the FDA previously authorized to use the Veritor system to detect other diseases. Those restrictions limit how many people might be able to get tested with the Veritor system.
Despite the limitations of the Veritor coronavirus test, BD is banking on high demand.
“We are ramping up larger manufacturing capacities at the existing BD plant in Suzhou, China, as well as by a contract manufacturer in Massachusetts,” the company told The Daily Beast in a statement. “We expect to manufacture up to 10 million tests from July through September, and ramp production to two million tests per week by the end of September.”
Following the FDA’s approval of the Veritor test for emergency use, BD shares rose more than 2 percent to $250.61 in early trading, according to The New York Times.
Got a tip? Send it to The Daily Beast here
Get our top stories in your inbox every day. Sign up now!
Daily Beast Membership: Beast Inside goes deeper on the stories that matter to you. Learn more.

 Yahoo News
Yahoo News 
