Men on hair-loss drug warned of sexual and psychiatric side effects

Men on a popular hair-loss drug have been warned of its potential serious sexual and psychiatric side effects by the drug regulator.
The Medicines and Healthcare products Regulatory Agency (MHRA) said men should be aware that finasteride has been linked to depression and suicidal behaviour as well as sexual dysfunction that in some cases continued beyond the treatment.
It has also ordered suppliers of finasteride to now include a patient alert card in packs of the drug to raise awareness of the risks.
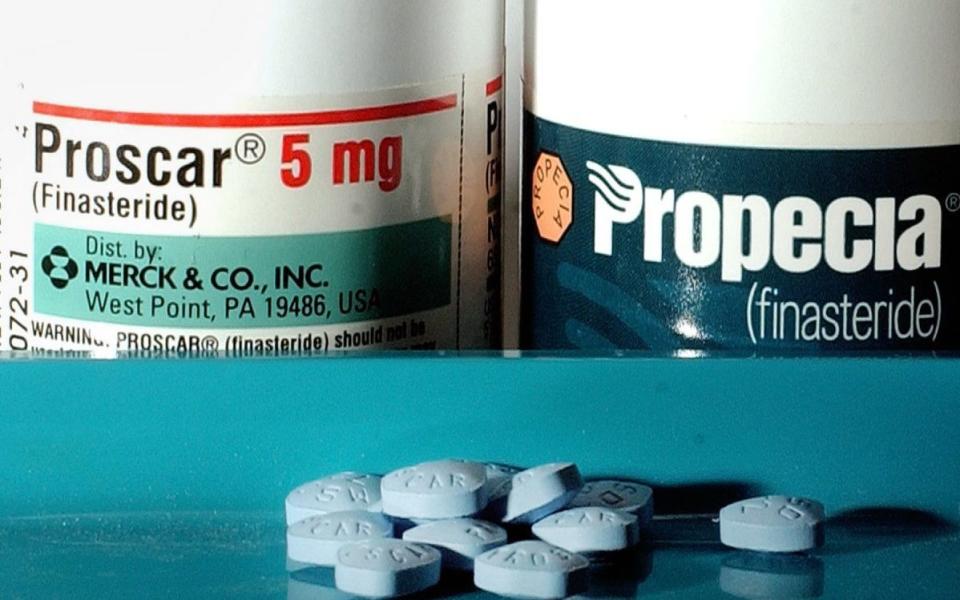
Finasteride, also known by brand names Proscar and Propecia, is used to treat benign enlarged prostate and male pattern baldness, but is only available on the NHS for the former.
It works by stopping the sex hormone testosterone from turning into another hormone called dihydrotestosterone (DHT), which can cause the prostate to grow, and hair to fall out.
An enlarged prostate is a common condition in men over the age of 50, affecting as many as three million, although many will not realise or need treatment until symptoms, such as frequent urination, develop.

King Charles underwent surgery for a benign enlarged prostate earlier this year when a cancer, not of the prostate, was found. It is not known whether he has ever taken finasteride.
It has been linked to mood disorders and sexual dysfunction previously, but now the MHRA has intervened after “a comprehensive review of the safety” of the drug.
The regulator said its Yellow Card scheme, designed to allow patients to report adverse effects of medicines, had received 281 reports of depressed mood disorders and suicidal or self-harm behaviours, as well as 426 reports related to sexual dysfunction.
It said an expert panel had reviewed all available evidence, including patients reporting symptoms of deteriorating mental health and sexual dysfunction, such as loss of libido and inability to achieve or maintain an erection.
In some cases sexual function did not return after stopping the drug, it said.
Dr Alison Cave, MHRA chief safety officer, said: “It’s crucial that patients are aware of vital information about the medicines they’re taking.
“The new patient alert cards aim to raise awareness among men taking finasteride about the potential for psychiatric and sexual side effects, so they can make an informed decision about their treatment and know what to do if they experience these side effects.”
She said patients should “remember to always read the leaflet inside the pack”.
The MHRA is recommending that patients inform their doctors of any personal history of depression or suicidal thoughts before taking the drug and stop the treatment “immediately” if they are taking it for hair loss and develop symptoms, or “speak to their doctor urgently” if it is for an enlarged prostate.
Sexual dysfunction should also be discussed with a doctor if symptoms develop.
The MHRA also encouraged patients to show the patient alert card to friends and family as they “may not notice changes to their mood” themselves.
There were around 4.2 million NHS prescriptions for the drug in 2023, which suggests around 350,000 a month were taking the daily 5mg pill for an enlarged prostate.
It is not known how many people get the drug for hair loss on a private prescription but some clinics have reported an increase. It is only licensed by the MHRA for hair loss in 18 to 41-year-olds, and the treatment of enlarged prostates.

 Yahoo News
Yahoo News 
