Hancock says there is no evidence AstraZeneca vaccine has caused blood clots
Matt Hancock has sought to reassure the public the Oxford/AstraZeneca vaccine is safe, insisting there is no evidence the coronavirus jab has caused blood clots after some European nations halted its rollout.
The Health Secretary urged the public to come forward and get the jab as the the European Medicines Agency (EMA) conducts a full scientific review – with France, Germany, Spain and Italy pausing their programmes.
The regulator, which approved the vaccine for the EU, says it currently “remains convinced” that the “benefits of this vaccine outweigh the risk”.
It is due to offer a further update on Thursday after several European countries halted its use due to reports of some people suffering blood clots following vaccination.
Mr Hancock told broadcasters: “The Oxford/AstraZeneca jab is safe, we know that over 10 million people have had it in this country, and that’s what the British regulator says but also the World Health Organisation and even the European regulator.
“We keep the effects of these vaccines under review all the time and we know that the Oxford/AstraZeneca vaccine is saving lives in the UK right now so if you get the call, get the jab.”
The Health Secretary added in an article for The Sun that “there is no evidence that vaccines caused these clots”.
His message came as the World Health Organisation (WHO) issued a new statement saying it was also evaluating the reports, but still believed the benefits of the AstraZeneca vaccine outweighed any risks.
Emer Cooke, the EMA’s executive director, told a press briefing on Tuesday there was “no indication” that the vaccine was the cause of the “very rare” reported blood clots.
“The number of thromboembolic events overall in vaccinated people seems not to be higher than that seen in the general population,” she added.
Ms Cooke said 30 cases of blood clots had been reported to the EMA by March 10 among almost five million people vaccinated, but additional cases had been reported over the weekend.
She said there would be an increase in the reporting of such cases due to the publicity surrounding the current reports.
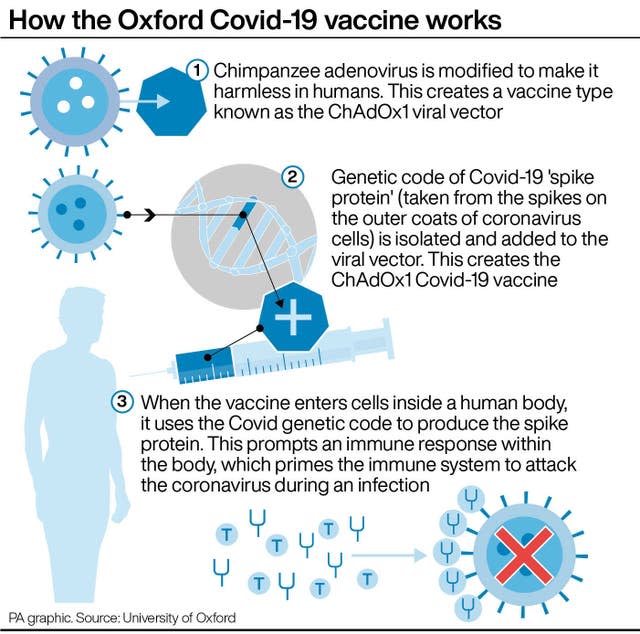
The EMA is looking at the incidence of blood clots and some reports of abnormally low levels of blood platelets among some people who have had the jab.
On Tuesday night, French prime minister Jean Castex suggested attitudes towards the AstraZeneca jab in the country were shifting after telling broadcasters he would accept a dose of the vaccine “as soon as the suspension is lifted”.
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) said it had received 30 reports of blood clots in people who had the Oxford/AstraZeneca jab and 38 reports associated with Pfizer/BioNTech in the UK up until February 28.
“Such reports are not proven side effects of the vaccine. Blood clots can occur naturally and are not uncommon,” a statement said.
On Tuesday afternoon, the WHO said there had been reports of clots in “very few people”, but “there are several aspects of these cases which require careful evaluation, including the age of patients, clinical features and severity of conditions”.
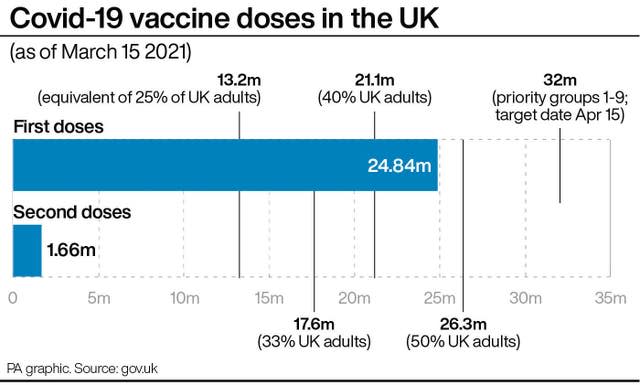
Earlier, the director general of Italy’s medicines authority, Nicola Magrini, told Italian daily newspaper la Repubblica that the decision by some European countries to suspend the rollout was a “political one”.
He said the vaccine was safe and added: “We got to the point of a suspension because several European countries, including Germany and France, preferred to interrupt vaccinations… to put them on hold in order to carry out checks. The choice is a political one.”
According to AstraZeneca, about 17 million people in the EU and the UK have received a dose of the vaccine, with fewer than 40 cases of blood clots reported to date.

 Yahoo News
Yahoo News 
