Russia to sell 100 million doses of controversial coronavirus vaccine to India

Following Russia’s claim to have the world’s first coronavirus vaccine, some 100 million doses are now being sold to India.
In a move that signals Moscow’s speed to up its plans to distribute the controversial vaccine – dubbed Sputnik V – abroad, it has agreed to supply the doses to Indian drug company Dr Reddy’s Laboratories.
The deal comes after the Russian Direct Investment Fund (RDIF) reached agreements with Indian manufacturers to produce 300 million doses of the vaccine in India, which is a major consumer of Russian oil and arms.
Dr Reddy's, one of India's top pharmaceutical companies, will carry out Phase III clinical trials of the vaccine in the country, pending regulatory approval, RDIF said in a statement.
However, the deliveries to India, where coronavirus cases have surged past five million, could begin before the end of this year.
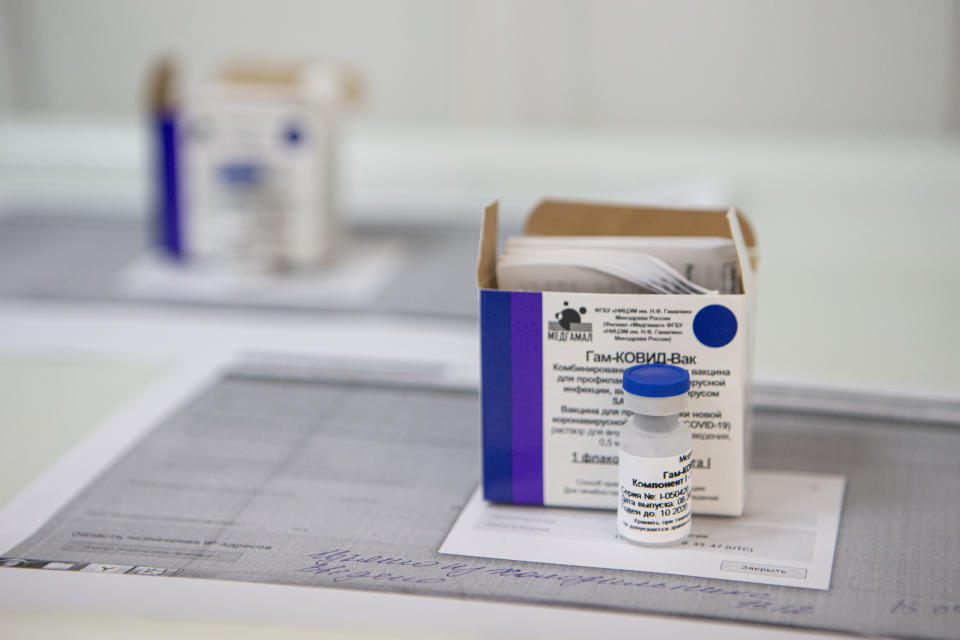
Vaccine scepticism
The Sputnik V vaccine has been met with controversy ever since Russian president Vladimir Putin said that it had been registered for use in August – and that one of his daughters has already been inoculated.
German health minister Jens Spahn said he was sceptical about the claims, warning they could ultimately “kill the acceptance” of vaccination as a weapon against the pandemic.
And last week, scientists from around the world signed an open letter expressing their concern that results of studies suggesting the vaccine was safe were “highly unlikely”.
In the letter, spearheaded by Dr Enrico Bucci from Temple University in Philadelphia, the scientists wrote that “the public’s extreme interest and expectations for an effective vaccine” should “motivate the scientific community to pay even more attention to the evidence”.
Nevertheless, a team of Russian scientists concluded that there were no serious safety concerns out of the 36 volunteers in trials up to 42 days after the vaccine was administered.
Writing in medical journal The Lancet, they added that the immune response was brought about in 40 volunteers within 21 days.
In response, medical experts from universities across the globe published an open letter on Italian site Cattivi Scienziati, accusing the Russian scientists of not providing “numerical data for all the experiments”.
Watch: What are the complications linked to the coronavirus?
They added that the research in The Lancet article pointed out “several different points of concern”.
Russia has dismissed the concern over its vaccine claims, describing the criticism as “absolutely groundless”.
Its health minister Mikhail Murashko told the Interfax news agency: "It seems our foreign colleagues are sensing the specific competitive advantages of the Russian drug and are trying to express opinions that... are absolutely groundless.”

UK vaccine progress
A potential vaccine developed in the UK could be ready as early as this year, after promising results were shown in the initial trials.
The UK government has signed a deal with pharmaceutical giants GlaxoSmithKline (GSK) and Sanofi Pasteur to secure 60 million doses of a potential coronavirus vaccine.

A further agreement has been signed with AstraZeneca and the University of Oxford for their jab, which could produce 100 million doses for the UK.
However, a recent poll found that only half of Britons would get a coronavirus vaccine if one was developed.
The Ipsos Mori survey of 2,237 adults found that 53% would be certain or very likely to take the vaccine, while one in six said they would definitely not get a vaccine, or it would be very unlikely.

China concerns
Meanwhile, China is inoculating tens of thousands of its citizens with experimental coronavirus vaccines, despite concerns among experts over the safety of drugs that have not completed standard testing.
China's approach goes against that of many Western countries, where experts have warned against authorising the emergency use of vaccines that have not completed testing, over concerns of potential side effects.
Coronavirus: what happened today
Click here to sign up to the latest news and information with our daily Catch-up newsletter

 Yahoo News
Yahoo News 


