India limits medicine exports after supplies hit by coronavirus
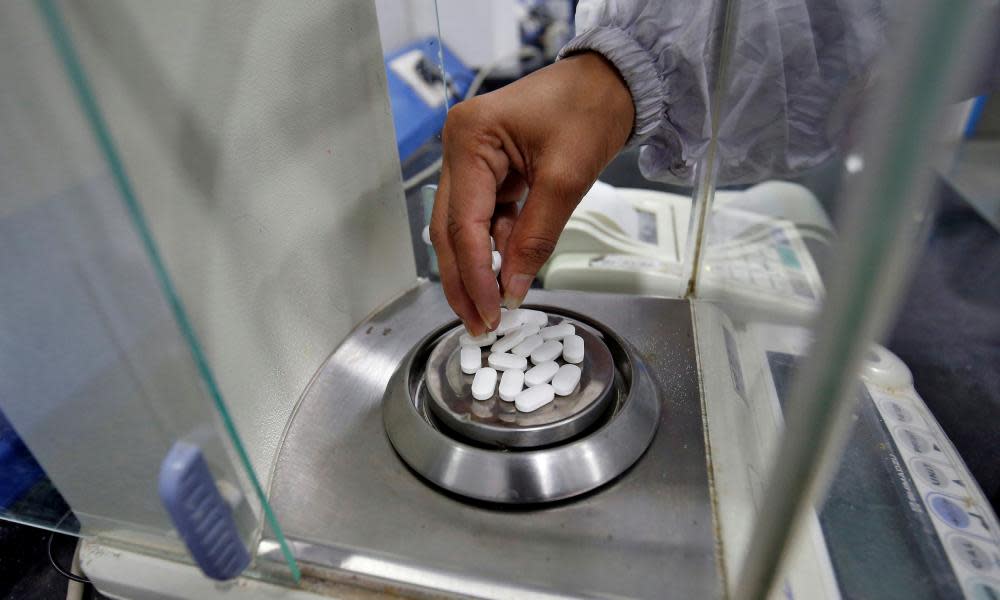
The coronavirus outbreak has led India to restrict the export of dozens of drugs including paracetamol and various antibiotics, leading to fears of a global shortage of essential medicines.
On Tuesday, concerns over supply chain shortages led the Indian government to place limits on the export of 26 pharmaceutical ingredients and the medicines and vitamins made from them.
The restricted drugs include paracetamol, antibiotics such as tinidazole and erythromycin, the hormone progesterone, which is used in the contraceptive pill, and and vitamins B12, B1 and B6. The drugs account for 10% of all India’s pharmaceutical exports.
India is one of the world’s largest producers and exporters of drugs, with the US and Europe heavily reliant on the supply.
India’s pharmaceutical companies source about 70% of their ingredients from Chinese factories, many of which have been shut for weeks owing to the coronavirus outbreak. The closure of airports in China has also impeded supplies reaching India.
The World Health Organization is recommending that people take simple precautions to reduce exposure to and transmission of the Wuhan coronavirus, for which there is no specific cure or vaccine.
The UN agency advises people to:
Frequently wash their hands with an alcohol-based hand rub or warm water and soap
Cover their mouth and nose with a flexed elbow or tissue when sneezing or coughing
Avoid close contact with anyone who has a fever or cough
Seek early medical help if they have a fever, cough and difficulty breathing, and share their travel history with healthcare providers
Avoid direct, unprotected contact with live animals and surfaces in contact with animals when visiting live markets in affected areas
Avoid eating raw or undercooked animal products and exercise care when handling raw meat, milk or animal organs to avoid cross-contamination with uncooked foods.
Despite a surge in sales of face masks in the aftermath of the coronavirus outbreak, experts are divided over whether they can prevent transmission and infection. There is some evidence to suggest that masks can help prevent hand-to-mouth transmissions, given the large number of times people touch their faces. The consensus appears to be that wearing a mask can limit – but not eliminate – the risks, provided it is used correctly.
While many Indian factories had stockpiled ingredients due to the lunar new year, those supplies are running low and most factories are covered only until the end of March.
Sahil Munjal, the vice-chair of the pharmaceutical export promotion council of India, said the restrictions could have a lasting impact on the global availability of commonly used drugs.
“In India we have maximum dependency on China, so if things do not improve in March and China’s factories do not reopen then it is going to be a big, big disruption in global logistics and in the market,” Munjal said.
He said the impending shortages were already pushing up prices. “If coronavirus goes on much longer, there will definitely be an impact on Indian plants and then 100% there will be a shortage of these medicines across the world. And right now it is very difficult to know what is happening in China, so we don’t know when the factories might open again.”
default
Indian pharmaceutical companies said they were still unclear what the restrictions entailed. More than 50% of India’s drugs are made for export, and many drugs produced in India cannot be sold on the domestic market.
Jatish Sheth, the director of Srushti Pharmaceuticals, said: “These are everyday products and the idea with the restrictions is to prevent a shortage in the country. But this will have a global impact.”
Sheth said pharmaceutical companies in India were worried about the situation. “If things in China start moving now, there might not be a very acute shortage,” he said. “But if they do not then it is going to be a serious problem.”
While some of the ingredients are also produced in factories outside China, the strict regulation of drug production means Indian factories cannot simply source them elsewhere.
In the US, Dr Stephen Hahn, the head of the Food and Drug Administration (FDA), told the Senate health committee on Tuesday that there was uncertainty over the impact India’s ban would have. “We’re working very closely to look at that list to assess how that will affect the medical supply chain,” he said.
India’s government urged people not to panic and said there were enough stocks of the medicines to last three months.
Kedar Upadhye, the chief financial officer of Cipla, one of India’s largest pharmaceutical companies, said: “Being aware of the dependence of pharmaceutical supply chain on China sourcing materials, we are making efforts to enhance our supply robustness and improve the forward cover for various materials. At this stage we don’t see any large disruption. However, we continue to watch the situation.”

 Yahoo News
Yahoo News 
