Moderna (MRNA) Up 20% on Upbeat Data From Cancer Jab Study
Shares of Moderna MRNA were up 19.6% on Dec 13 after management announced that the phase IIb KEYNOTE-942 study evaluating its personalized cancer vaccine (PCV) candidate mRNA-4157/V940 achieved its primary endpoint of recurrence-free survival (RFS). The PCV candidate is being developed in collaboration with Merck MRK.
The KEYNOTE-942 study evaluated the combination of mRNA-4157 and Merck’s blockbuster cancer drug Keytruda against Keytruda alone as an adjuvant treatment for stage III/IV melanoma patients with a high risk of recurrence following complete resection.
Data from the study showed that the mRNA-4157/Keytruda combination exhibited a statistically significant and clinically meaningful reduction in the risk of disease recurrence or death by 44%. The study’s results also suggest that a personalized neoantigen approach may benefit melanoma patients.
Based on the above data, Moderna and Merck plan to discuss these results with regulatory authorities and also intend to start a phase III study in melanoma patients next year. Detailed results are also expected to be presented at a future medical meeting.
Unlike other vaccines that are uniformly designed to treat all patients, the PCV vaccine aims to bring individualized treatment to cancer patients. mRNA-4157 is tailored for each patient based on the unique mutational signature of a patient's tumor. Per a Reuters article, it currently takes eight weeks to manufacture a PCV vaccine though management hopes to reduce this time frame by half.
The companies also plan to expand the PCV vaccine to cancer indications other than melanoma.
Unlike traditional vaccines, mRNA-based vaccines teach the body how to make a specific protein that can help your immune system prevent or treat certain diseases. The COVID-19 pandemic has further demonstrated the significant potential of mRNA-based therapeutics. By way of COVID vaccines, Moderna and Pfizer have generated immune responses against the virus at record-high levels compared to traditional protein-based and adeno-based vaccines.
In the year so far, the stock has lost 22.2% compared with the industry’s 18.5% decline.
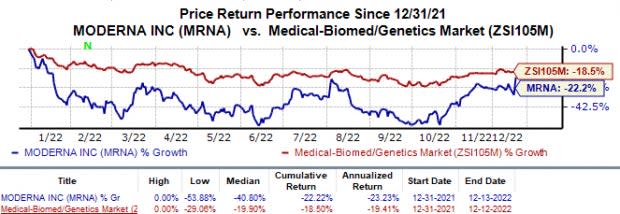
Image Source: Zacks Investment Research
Merck’s Keytruda is already approved for multiple melanoma indications. Keytruda is approved by the FDA as an adjuvant treatment for patients aged 12 years and older with stage IIB/IIC/III melanoma patients following complete resection. It is also approved for treating patients with unresectable or metastatic melanoma.
Merck and Moderna entered a strategic partnership in 2016 to develop and commercialize mRNA-based personalized vaccines for the treatment of various types of cancer. Earlier in October, Merck exercised its option to develop the PCV vaccine with Moderna. In consideration for exercising the option, Merck paid $250 million to Moderna. Per the terms of collaboration, the companies will share costs and profits equally.
Following the end of the pandemic in the United States and the government encouraging citizens to resume pre-pandemic activities, Moderna is rapidly advancing its non-COVID pipeline and reducing dependence on its COVID-19 vaccine sales. Vaccine sales are seeing a declining trend and are expected to fall further in future quarters.
Currently, Moderna has three late-stage candidates — mRNA-1647, mRNA-1345 and mRNA-1010 — in its pipeline, which are being developed as cytomegalovirus (CMV) vaccine, respiratory syncytial virus (RSV) vaccine and influenza vaccine, respectively. A successful development of any or all of these candidates and potential commercialization will help lower the company’s dependence on a single product for revenue.
Moderna, Inc. Price
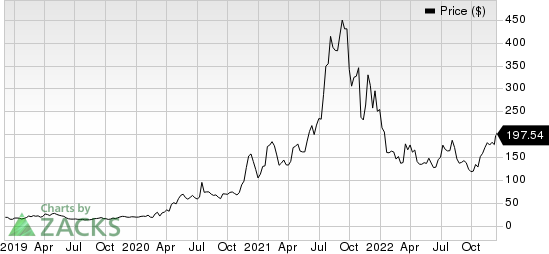
Moderna, Inc. price | Moderna, Inc. Quote
Zacks Rank & Stocks to Consider
Moderna currently carries a Zacks Rank #3 (Hold). A couple of better-ranked stock in the overall healthcare sector includes BioNTech BNTX and Kamada KMDA. While Kamada sports a Zacks Rank #1 (Strong Buy) at present, BioNTech carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 30 days, estimates for Kamada’s 2022 loss per share have narrowed from 14 cents to 7 cents. During the same period, the earnings estimates per share for 2023 have risen from 26 cents to 42 cents. Shares of Kamada have declined 30.2% in the year-to-date period.
Earnings of Kamada beat estimates in two of the last four quarters and missed the mark twice, witnessing a negative earnings surprise of 62.50%, on average. In the last reported quarter, Kamada’s earnings beat estimates by 433.33%.
In the past 30 days, estimates for BioNTech’s 2022 earnings per share have risen from $34.47 to $35.38. During the same period, the loss estimates per share for 2023 have narrowed from $16.97 to $17.53. Shares of BioNTech have lost 30.9% in the year-to-date period.
Earnings of BioNTech beat estimates in three of the last four quarters and missed the mark once, witnessing a surprise of 58.99% on average. In the last reported quarter, BNTX delivered an earnings surprise of 92.35%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Kamada Ltd. (KMDA) : Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report

 Yahoo News
Yahoo News 