Student doctors and vets could administer Covid-19 vaccine, under government plans to rush out doses


Students, dentists and even vets could be recruited to administer a new coronavirus vaccine, under plans to rush out millions of doses.
The government announced on Friday it wants to change the law to dramatically increase the size of the workforce entitled to issue injections.
Ministers also plan to alter the rules on legal liability to make it harder for patients to sue healthcare workers if something goes wrong.
The government is currently backing six different vaccine candidates, and has placed orders for 340 million doses, to be used if and when a drug passes its safety and efficacy hurdles.
Although a senior health official said on Friday he thought the chances of an effective vaccine coming online this side of Christmas were “small but plausible”, planners are trying to look ahead to prevent any bottlenecks in delivery when a drug does become widely available.
As well as greatly expanding the workforce of people able to administer the jab, plans also includes potentially allowing a vaccine to be used before it is fully licensed by the regulator.
The Department of Health and Social Care insisted that this is mainly of bureaucratic significance - one consequence being to increase the financial risk to the drug manufacturers - and that no corners would be cut on safety.
Currently doctors, nurses and pharmacists administer vaccines, such as the annual flu jab.
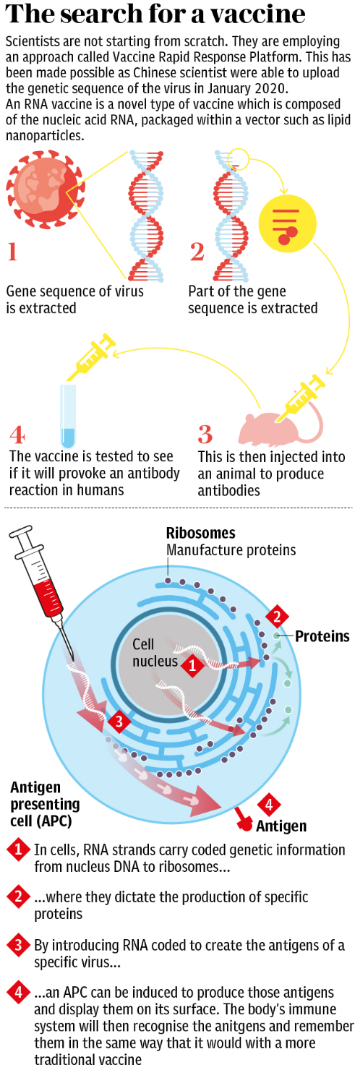
The graphic below shows the steps on the way to creating a vaccine.
Deputy chief medical officer Professor Jonathan Van-Tam said: "We are making progress in developing Covid-19 vaccines which we hope will be important in saving lives, protecting healthcare workers and returning to normal in future.
"If we develop effective vaccines, it's important we make them available to patients as quickly as possible but only once strict safety standards have been met.
"The proposals consulted on today suggest ways to improve access and ensure as many people are protected from Covid-19 and flu as possible without sacrificing the absolute need to ensure that any vaccine used is both safe and effective."
Launched on Friday, the consultation looking at amending the Human Medicine Regulations 2012 will last just three weeks.
If a vaccine is discovered before 2021, the proposals will bolster existing powers that allow the MHRA to consider approving its use, before a full product licence is granted.
This is provided it is proven to be safe and effective during clinical trials.
The measures are necessary because during the transition period, a potential Covid-19 vaccine must be granted a licence by the European Medicines Agency (EMA).
The regulations will permit the MHRA to consider giving temporary authorisation, allowing patients to benefit while it undergoes the full licencing process.
Dr Doug Brown, Chief Executive of the British Society for Immunology, said: “Providing vaccination services and ensuring these services are accessible and promote high uptake is an extremely complex task which requires significant resources.
"A focus on increasing the vaccination workforce, particularly with regards this year’s extended flu vaccination programme and any potential future COVID-19 vaccine, is a positive move."

 Yahoo News
Yahoo News 
